Adapter Chimeric Antigen Receptor
Metastatic disease remains one of the greatest challenges for the treatment of tumors. The aim of our study was the preclinical evaluation of the efficacy of adapter chimeric antigen receptor (AdCAR)-modified NK-92 cells as a possible treatment strategy for various types of bone metastatic cancers. We confirmed that AdCAR NK-92 cells successfully induce tumor cell lysis in bone metastasis cell lines derived from breast, renal, and colorectal carcinoma as well as melanoma in a specific and controllable manner, thereby establishing a potent cell product with universal applicability and rapid clinical translation potential for the treatment of solid tumors, including metastases.
Summary
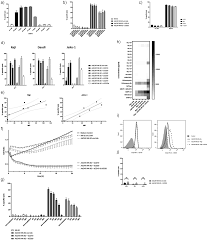
Background: Since the metastatic spread of solid tumor cells often results in a fatal outcome for most cancer patients, new individualized targeted immunotherapy approaches are urgently needed. Methods: Here, we established cell lines from four bone metastases from different tumor entities. We assessed AdCAR NK-92-mediated cytotoxicity in vitro in standard cytotoxicity assays as well as 3D spheroid models
AdCAR-engineered NK-92 cells successfully demonstrated distinct and specific cytotoxic potential targeting different tumor antigens expressed on cell lines established from bone metastases of breast, renal, and colorectal carcinomas, as well as melanomas. During this process, AdCAR NK-92 cells produced a host of NK effector molecules as well as pro-inflammatory cytokines. Additionally, AdCAR NK-92 showed enhanced cytotoxicity in 3D spheroid models that can recapitulate in vivo architecture, thereby bridging the gap between in vitro and in vivo models.
